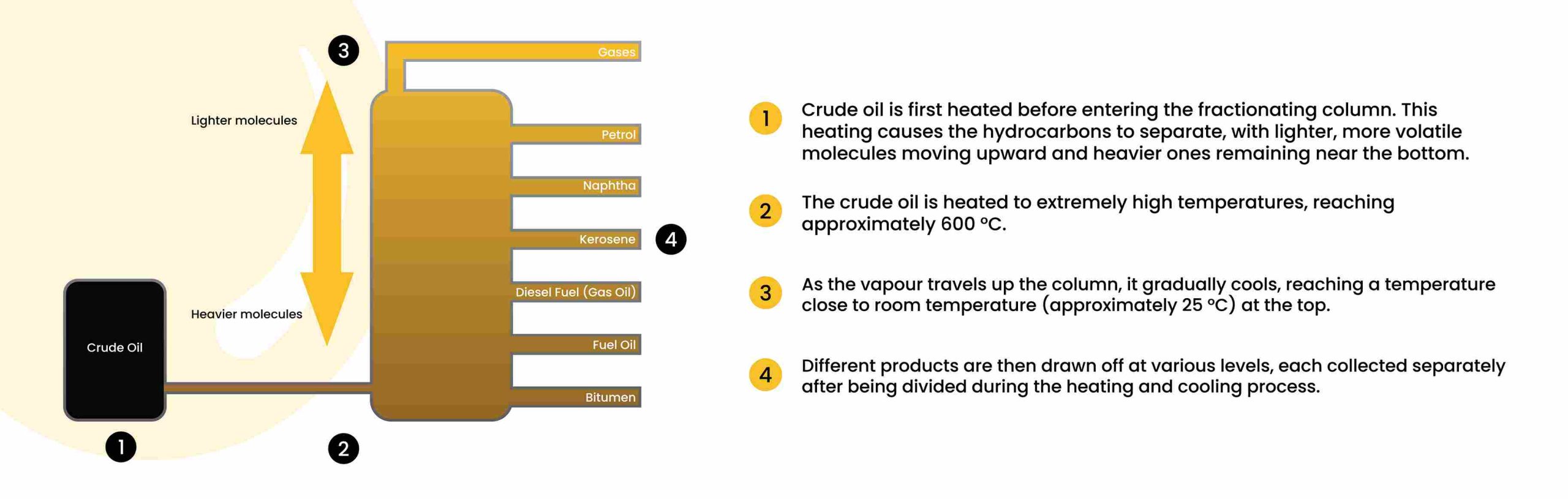
Crude oil is separated by fractional distillation, where it is heated and its vapours rise through a distillation column. The lighter hydrocarbons, such as LPG, petrol and naphtha, condense at the top, while middle fractions like kerosene and diesel condense further down. Heavier products, including lubricating oils, heavy fuel oils and bitumen, remain at the bottom. This process ensures each fraction is collected at its specific boiling point range and used for fuels, heating, or industrial applications.
Introduction: From Black Goo to Everyday Fuel
Crude oil is often regarded as one of the most valuable natural resources on Earth. Yet, in the state it is extracted, crude oil is not directly usable for most of our everyday needs. What transforms this dark, viscous fluid into petrol, diesel, kerosene, lubricants, bitumen, and more is a methodical refining process, with fractional distillation usually taking pride of place as the first and vital step.
In this guide, we’ll explore what crude oil is, how fractional distillation works, the various fractions extracted, associated challenges, and ongoing innovations. Whether you are an energy enthusiast, a student, or someone curious about how your car gets its petrol, this blog offers insight into the heart of the petroleum industry.
What Is Crude Oil?
Crude oil, sometimes known simply as petroleum, is a naturally occurring mixture of hydrocarbons (molecules made of hydrogen and carbon). It is formed over millions of years from organic matter, remains of plants, algae, and microorganisms, that have been subjected to heat, pressure, and geological changes deep beneath the Earth’s surface.
Chemically, crude oil comprises a vast variety of hydrocarbon chains: some are short, volatile, and gaseous or light liquids; others are long, heavy, viscous, and complex. It may also contain impurities such as sulfur, nitrogen, oxygen compounds, metals, and salts.
In its raw form, crude oil is black or dark brown, thick, and sometimes waxy, making it unsuitable for direct use in engines or heating applications. Thus, refinement is essential.
Some key points about crude oil:
- Its composition can differ greatly depending on the source or oil field (light crude, heavy crude, sweet crude, sour crude, etc.).
- It needs pre-treatment before distillation (e.g. desalting to remove salts and water).
- Its physical properties (viscosity, density, boiling range) influence how easily it can be refined.
Fractional Distillation: The Core Separation Process
Fractional distillation is the process by which a mixture (in this case, crude oil) is separated into various fractions, or groups of hydrocarbons, according to their different boiling points. The principle is that when you heat a mixture, the components with lower boiling points vaporise first. As vapours rise in a column, they cool and condense at various heights depending on their volatility.
In oil refining, fractional distillation is typically performed in an atmospheric distillation unit (also known as a crude distillation unit), which operates at or slightly above atmospheric pressure.
Steps in Fractional Distillation
Preheating and Desalting
Before entering the distillation tower, crude oil is heated (often via heat exchange with outgoing products) and passed through a desalter to remove salts and water. This is essential to prevent corrosion and fouling in downstream equipment.
Heating to Vaporisation
The preheated crude is further heated in a furnace to a temperature (often around 350-400 °C or more) that causes most of it to vaporise (but not always all; some heavy residue remains liquid).
Entry into the Fractionating Column
The heated mixture enters the bottom of a tall vertical column (also called a fractionating column, distillation tower or column). Inside, it experiences a temperature gradient, with the hottest temperatures at the base and cooler temperatures towards the top.
Vapour-Liquid Equilibria and Stagewise Condensation
As vapour rises, the mixture cools. Heavier (higher boiling) components condense and fall back down; lighter ones stay in vapour form and ascend. Each tray or plate (or packing) inside the column provides an interface for vapour to condense and liquid to re-vaporise, gradually enriching fractions in specific hydrocarbon ranges.
Collection of Fractions (Side Cuts)
At various levels of the column, different fractions are drawn off (so-called “side cuts”). At the top, the lightest vapours (gases) emerge; further down, condensates such as petrol, naphtha, kerosene, diesel, and heavier oils are tapped off; finally, the bottom yields the residual, heavy oils and bitumen.
Cooling and Further Processing
The separated streams are cooled (via condensers) to convert vapours to liquids, and then often undergo further treatment, such as purification, cracking, reforming, and blending, to meet product specifications.
Thus, fractional distillation is both a physical and thermodynamic separation method.
The Distillation Column: Design and Operation
The design of the distillation column is central to efficiency, yield and purity. Some key features:
- Height and number of theoretical plates: Taller columns and more trays or packing increase separation by providing more contact stages.
- Temperature gradient control: Precise control of temperatures along the column is vital to ensure correct condensation of each fraction.
- Reflux ratio: This is the portion of condensed liquid returned down the column versus withdrawn as product. Adjusting reflux allows tradeoffs between purity and throughput.
- Feed point location: The location where heated crude enters the column affects separation efficiency.
- Material and corrosion resistance: Due to the high temperatures and corrosive compounds (especially sulfur), the column and trays must be constructed from robust materials.
Columns can also be equipped to operate under vacuum or combined with vacuum distillation units to distil heavy fractions at lower pressures, reducing the risk of thermal cracking. While atmospheric distillation handles the bulk of separation, vacuum distillation is used for high-boiling, heavy residues.
A wide array of products emerges from fractional distillation. Below is a breakdown of typical fractions, their boiling ranges (approximate), and common uses.
Fraction | Boiling Range / Conditions | Characteristics | Common Uses |
Gases (e.g. methane, ethane, propane, butane) | ~ < 30-60 °C | Very volatile, light | LPG, petrochemical feedstock, heating, cooking |
Petrol / Gasoline | ~ 40-200 °C | Low to moderate volatility, blends of C5–C12 hydrocarbons | Fuel for petrol (gasoline) engines |
Naphtha | ~ 60-100 °C (or overlaps with petrol range) | Light liquid | Petrochemical industry, feedstock for cracking |
Kerosene / Paraffin | ~ 150-250 °C | Moderate volatility | Jet fuel, domestic heating, lamps |
Diesel / Gas oil | ~ 200-350 °C | Less volatile, higher density | Diesel vehicles, generators, heating |
Heavy fuel oils / Bunker fuels | ~ 300-400+ °C | High boiling point, viscous | Marine fuels, industrial burners |
Lubricating oil | ~ 300-400+ °C | Viscous, stable | Engine oil, greases, mechanical lubrication |
Residue, bitumen / asphalt | > ~500-600 °C | Thick, heavy, non-volatile | Road surfacing, roofing, asphalt |
Below is a more narrative description of each:
Top Distillates (Gases, Petrol, Naphtha)
At the top of the column, the lightest hydrocarbons condense first. Petroleum gases (like propane and butane) emerge at low temperatures (≈ 25 °C) and are used in LPG or as feedstock for chemical processes. Petrol (gasoline) is one of the most demanded products, often as much as 50–60% of refined output. Naphtha sits just below petrol in boiling range, and is a major feedstock for the petrochemical industry (plastics, solvents, etc.).
Middle Distillates (Kerosene, Diesel, Paraffin)
These condense lower down. Kerosene (or “paraffin oil” in British usage) condenses between ~175–250 °C and is used as heating oil and jet fuel (aviation grades). Diesel emerges around 250–350 °C and is heavily used in vehicles, industrial engines, and heating systems. Some paraffin waxes, formed from heavier middle distillate fractions, are processed for use in candles, wax products, and personal care items.
Lower Distillates & Residue (Lubricants, Heavy Fuels, Bitumen)
Lower in the column, heavier, non-volatile compounds such as lubricating oils, greases, and heavy fuel oils (bunker fuels, marine oils) are collected. The bottom residue, which may remain after extraction of all lighter fractions, is often bitumen or asphalt, used in road surfaces, roofing and construction.
Because market demand for lighter fuels (petrol, diesel) often exceeds the proportion produced by straight distillation, downstream processes such as cracking and reforming are applied to convert heavier fractions into lighter ones.
Cracking, Reforming and Further Processing
Fractional distillation is a physical separation process; it does not alter the chemical structure of hydrocarbons. However, the raw distillates are rarely the optimal end products. Hence, additional refining steps convert, reshape or purify these fractions:
Cracking
Breaking down larger, heavier hydrocarbon molecules into smaller, more useful ones (e.g. turning heavy fuel oil into petrol or diesel). It can be achieved thermally (through high heat) or catalytically.
Reforming
Rearranging hydrocarbon molecules (e.g. converting naphtha into high-octane petrol compounds).
Hydrotreating / Desulphurisation
Removing sulfur, nitrogen, metals and other impurities to meet environmental and fuel quality standards.
Blending and Additives
Combining different fractions or adding performance agents (detergents, anti-knock additives) to meet specifications (e.g. petrol octane rating, diesel cetane number).
These processes are crucial in ensuring that the fuels we burn or the lubricants we use meet rigorous performance, safety, and emission requirements.
Challenges & Key Considerations
While fractional distillation is well-established, it is not without its technical, economic, and environmental challenges. Some of these include:
Energy Intensity
Heating large volumes of crude oil to very high temperatures demands vast amounts of energy, often derived from fossil fuels, which contributes to greenhouse gas emissions and high operational costs.
Emissions & Environmental Controls
The process can release volatile organic compounds (VOCs), sulphur oxides, nitrogen oxides and other pollutants. Managing these emissions, along with handling wastewater, is crucial for ensuring regulatory compliance and maintaining community health.
Corrosion & Fouling
High temperatures, sulfur compounds, salts and other impurities can corrode metal surfaces. Organic or inorganic deposits (fouling) can reduce heat transfer efficiency and clog equipment, leading to decreased performance.
Feedstock Variability
Different crude oils have wildly different compositions. A refinery must adjust operating conditions to handle changing crude grades (light, heavy, sour, sweet) without compromising efficiency.
Economic Balance of Yield vs Purity
Optimisation involves tradeoffs: pushing for high purity may reduce yield or increase energy cost; aiming for maximum yield might degrade quality or require costly downstream processing.
Residue Handling
Heavy residual fractions (bitumen, asphalt) must find suitable markets, or else their storage and disposal become costly.
As the industry evolves, refiners are striving to overcome these challenges via technological innovation.

Innovations and the Future of Fractional Distillation
To address efficiency, costs and sustainability, the petroleum industry is innovating on several fronts:
Heat Integration and Waste Heat Recovery
By recapturing heat from outgoing streams to preheat input, refineries reduce net energy requirements.
Advanced Materials and Coatings
Corrosion-resistant alloys, protective linings and coatings help prolong equipment life and minimise downtime.
Automation, Sensors & Real-Time Control
Modern sensors, computer control systems, and AI/ML (machine learning) can monitor temperatures, flow, pressures, and composition in real-time, enabling finer adjustments and predictive maintenance.
Process Intensification
Novel column designs, more efficient packing, and compact reactor-distillation integrations may reduce footprint and increase efficiency.
Lower Carbon and Alternative Feedstocks
Some refineries experiment with co-processing bio-oils, waste plastics or other alternative hydrocarbons to gradually reduce reliance on pure crude oil.
Real-World Example: How a Brand May Appear in the Supply Chain
Imagine a fuel supplier, such as 123 Oil, receiving refined products from a refinery. While they don’t themselves run the distillation towers, they depend on the quality, consistency, and specifications of the refined products (petrol, diesel, lubricants, etc.). Their supply contracts, storage, blending capacity, logistics and quality assurance are all downstream consequences of how well that fractional distillation and refining process was executed upstream.
Why This Matters: Applications and Impacts
- The petrol and diesel that power cars, buses, trucks, ships, and trains originate (in part) from fractions distilled in that tower.
- Aviation & Heating. Jet fuel is largely derived from kerosene-based fractions, while domestic or industrial heating typically utilises kerosene or gas-oil
- Industrial & Chemical Feedstocks. Many petrochemicals, plastics, solvents, detergents, are derived from lighter-distilled fractions (naphtha, LPG).
- Lubrication & Machinery. Engines, turbines, gearboxes, and mechanical systems require specially processed lubricants derived from heavier fractions of crude oil.
- Construction & Infrastructure. Bitumen and asphalt for roads and roofing are derived from the bottom residue of crude oil distillation.
Thus, fractional distillation is foundational to modern industrial society.
Conclusion
Fractional distillation is not merely a textbook concept, it is the workhorse of the oil refining world. Through careful heating, vapour rise, condensation, and side-cut extraction, crude oil is broken down into hundreds of useful products. Yet, the process is not static: refiners continuously adapt column designs, energy recovery systems, automation and downstream processes to meet evolving demands for efficiency, environmental compliance, and lower carbon footprints.
Understanding how crude oil is transformed, from its raw subterranean origin to the petrol in your vehicle’s tank or the asphalt beneath your feet, gives deep appreciation for the engineering, chemistry and logistics involved. Whether you’re in the energy sector, an engineering student or simply fuel-curious, the story of crude oil and fractional distillation is central to the modern world.
Frequently Asked Questions
Fractional distillation is the process of heating crude oil and separating it into useful products, such as petrol, diesel, kerosene, and bitumen, based on their boiling points.
Crude oil in its raw state is not usable. Refining separates it into fractions that provide fuels, lubricants, and raw materials for plastics and other products.
Common products include petrol, diesel, kerosene, LPG, lubricating oil, heavy fuel oil, and bitumen, each of which is collected at different levels in the distillation column.
The column uses a temperature gradient: lighter hydrocarbons rise and condense near the top, while heavier fractions condense lower down or remain at the bottom.
The heavier fractions become products like lubricating oils, heavy fuel oils, or bitumen, and some are cracked into lighter, more useful fuels such as petrol or diesel.